在費森尤斯醫療保健公司Xenios,我們為患有心髒和/或肺衰竭的患者提供治療。基於對人類生理的全麵理解,我們開發了對危重患者的生存至關重要的智能技術解決方案。
我們提供在一個技術平台上結合心肺支持的解決方案。它支持肺部和心血管係統的氣體交換,以維持患者的生命。因此,醫生在診斷和治療過程中獲得了更重要的治療時間。
Xenios是費森尤斯醫療保健的一部分,費森尤斯醫療保健是全球領先的為腎髒疾病患者提供產品和服務的供應商。我們是費森尤斯醫療保健在重症監護領域的產品組合的補充,並繼續塑造體外多器官支持的未來。
在日常工作中,我們都樂於助人。為病人。在全球範圍內。每一天。
訪問Xenios網站:
www.xenios-ag.com

在社交媒體上關注我們吧!
產品
-
local Xenios AG representative.
Also, this information is not intended for an audience within the USA. If your are from the USA, please visit the corresponding Fresenius Medical Care website here.
"> -
• x.ellence, a multi-layer coating for stable bio- and hemocompatible surfaces• Two different sizes and three different configurations for the full range of extracorporeal respiratory support from efficient CO2 removal to full oxygenation">
For your information: Not all products are cleared or available for sale in all countries. For further information on country-specific regulations, please contact your local Xenios AG representative.
Also, this information is not intended for an audience within the USA. If your are from the USA, please visit the corresponding Fresenius Medical Care website here.
-
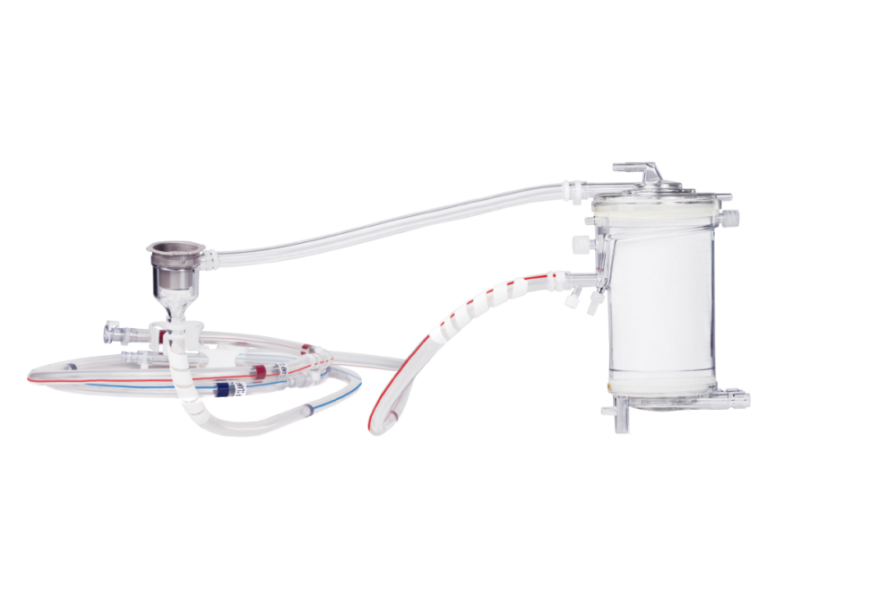
Extracorporeal organ support for enhanced patient well-being
Dedicated technology combines both heart and lung support in one single platform – a platform that is universally usable, individual, safe, and compatible with applications of our parent company, Fresenius Medical Care.
Universal - Wide spectrum of extracorporeal support
• Therapies for heart and lung assist on one single platform
• From neonates up to adults
• From partial CO2 removal to full oxygenation
• VV-, VA-applications: ICU, operating theater, cardiac catheterization lab
Reliable - Reliable technology and intuitive handling
• Easy therapy-driven interface
• Integrated Pressure Sensors (IPS)
• Dual power: AC power and battery pack
• Full alarm history on screen
Effective - Highly sophisticated pump technology
• Diagonal pump with broad flow range allows for individual settings
• Fine adjustments of flow rate even below 0.5 l/min
• Optional pulsatile flow
• More safety by p1-limiter monitoring, zero-flow mode and autopilot"For your information: Not all products are cleared or available for sale in all countries. For further information on country-specific regulations, please contact your local Xenios AG representative.
Also, this information is not intended for an audience within the USA. If your are from the USA, please visit the corresponding Fresenius Medical Care website here.
"> -
Elementary features:
• Proven reliability and safety
• Certified for 29 days application period
• Matching pump sizes for ¼ "" or 3/8"" tubing
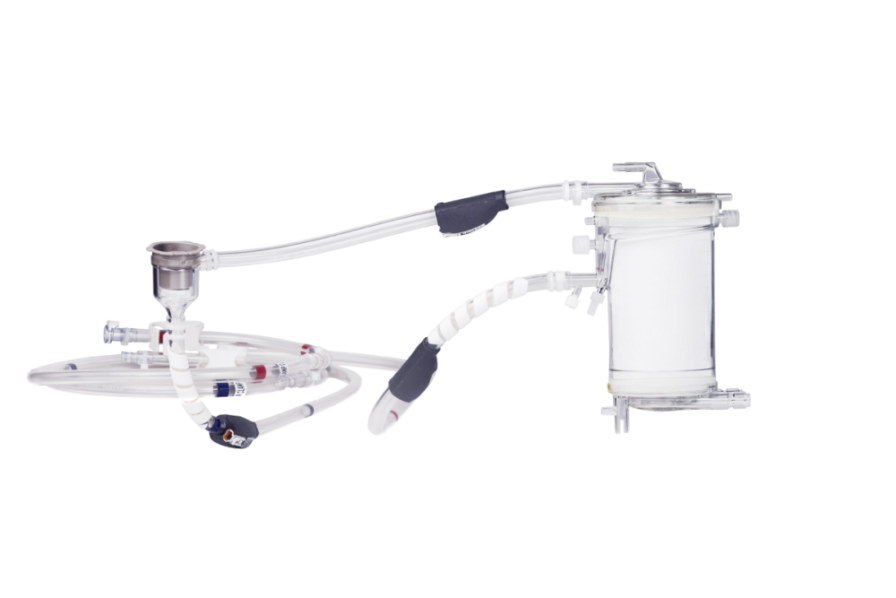
• x.ellence, a multi-layer coating for stable bio- and hemocompatible surfaces
• Fine adjustments of the flow rate possible
Available in three different sizes:
• iLA activve MiniLung petite kit
• iLA activve MiniLung kit
• iLA activve XLung kit"For your information: Not all products are cleared or available for sale in all countries. For further information on country-specific regulations, please contact your local Xenios AG representative.
Also, this information is not intended for an audience within the USA. If your are from the USA, please visit the corresponding Fresenius Medical Care website here.
"> -
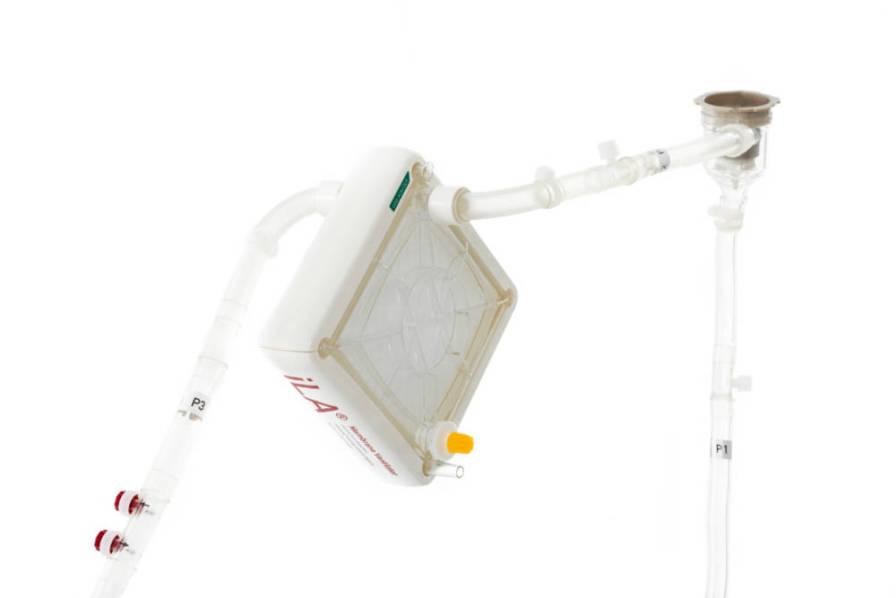
• CRRT connection
• DP3 pump head
• Fine adjustments of the flow rate possible
• Safe integrated pressure sensors (IPS)
- No air aspiration
- No risk of clotting
- No regular rinsing needed
- No hemodilution in pediatric patientsFor your information: Not all products are cleared or available for sale in all countries. For further information on country-specific regulations, please contact your local Xenios AG representative.
Also, this information is not intended for an audience within the USA. If your are from the USA, please visit the corresponding Fresenius Medical Care website here.
"> -
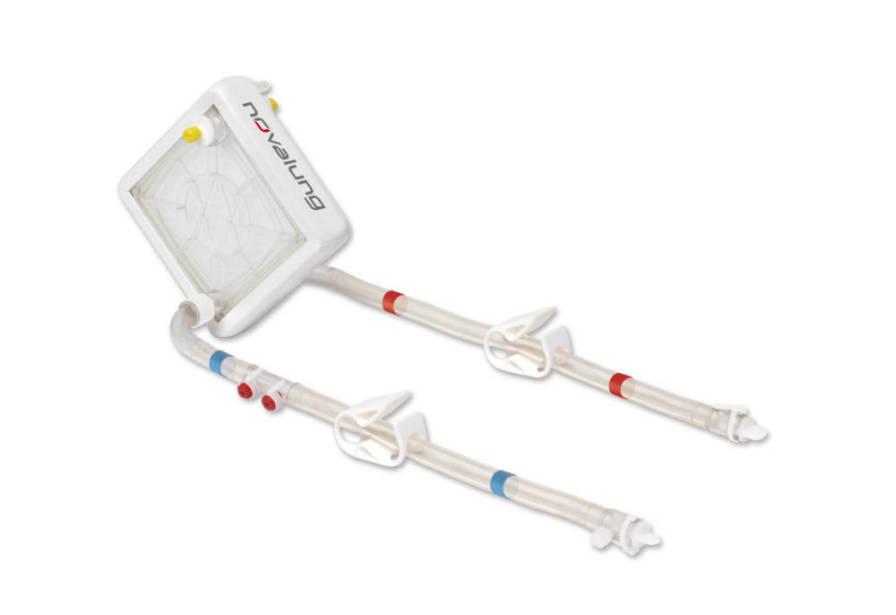
Advantages:
• Reliable efficacy – efficient CO2 removal
• x.ellence, a multi-layer coating for stable bio- and hemocompatible surfaces
• Easy handling
• One vascular access for iLA membrane ventilator and CRRT thanks to integrated CRRT connector"For your information: Not all products are cleared or available for sale in all countries. For further information on country-specific regulations, please contact your local Xenios AG representative.
Also, this information is not intended for an audience within the USA. If your are from the USA, please visit the corresponding Fresenius Medical Care website here.
"> -
For your information: Not all products are cleared or available for sale in all countries. For further information on country-specific regulations, please contact your local Xenios AG representative.
Also, this information is not intended for an audience within the USA. If your are from the USA, please visit the corresponding Fresenius Medical Care website here.
"> -
For your information: Not all products are cleared or available for sale in all countries. For further information on country-specific regulations, please contact your local Xenios AG representative.
Also, this information is not intended for an audience within the USA. If your are from the USA, please visit the corresponding Fresenius Medical Care website here.
">
事件

新聞
Xenios AG在中國獲得ECMO設備批準
2021年7月13日
費森尤斯醫療保健公司Xenios A6已獲得中國國家藥品監督管理局(NMPA)批準,用於ECMO治療的Xenios控製台和患者包。今年5月初,費森尤斯醫療保健公司Xenios AG獲得了在中國銷售兩套患者工具包的批準。這是在NMPA批準Xenios控製台之後…閱讀更多
Xenios AG宣布董事會改組
2021年3月31日
費森尤斯醫療保健公司的心肺業務部門Xenios AG的管理委員會已經重組。從2021年1月31日起,前執行董事會主席Andreas Terpin博士已離開公司。管理委員會新的高級三人組將延續費森尤斯醫療集團在體外…閱讀更多
iLA膜呼吸機使36歲婦女在肺移植前存活143天
2020年8月31日
布拉格綜合大學醫院的醫生挽救了一名36歲婦女的生命,她是一名兩歲孩子的母親,急需進行肺移植手術。在一種名為iLA膜呼吸機的設備的支持下,她在沒有完全工作的肺的情況下存活了令人難以置信的143天。iLA膜呼吸機是體外呼吸機…閱讀更多
ECMO成為歐洲抗擊COVID-19的關鍵療法——Xenios遊戲機成為救星
2020年4月30日
隨著冠狀病毒的傳播和COVID-19在歐洲的感染進一步增加,ECMO治療被證明是重症患者的一個必要選擇。Xenios AG是費森尤斯醫療保健的子公司,提供ECMO控製台,可用於治療嚴重肺炎和ARDS肺…閱讀更多
Novalung係統獲得美國FDA批準
2020年2月26日
美國FDA(食品和藥物管理局)已經批準費森尤斯醫療保健北美公司和費森尤斯醫療保健公司Xenios 510(k),用於長期使用其novvalung係統治療急性肺衰竭或急性心肺衰竭患者。FDA在周五晚上宣布了這一消息。經過II級批準,Novalung…閱讀更多
合並:Novalung完全集成到Xenios中
2019年9月02日
專注於呼吸衰竭解決方案的Novalung GmbH於2019年8月30日被Xenios AG收購。因此,流程將被簡化,該公司將與母公司費森尤斯醫療保健(Fresenius Medical Care)更加緊密地聯係在一起。諾瓦隆成立於2003年,專注於呼吸衰竭的治療....閱讀更多
同步心血管支持係統已準備上市
2019年7月10
Xenios AG公司總結了其首個i-cor同步心髒輔助係統的臨床研究,證明了同步心血管支持係統的安全性和可行性。在“SynCor”試驗期間,一項多中心研究在9個不同的德國診所治療了47名患者,使用一種創新的搏動、心髒同步循環支持……閱讀更多
重新啟動Xenios網站——現在與母公司費森尤斯醫療保健公司的網站協調一致
2019年08年5月
Xenios AG是體外心肺支持技術的先驅,它已經重組了網站。Xenios AG自2016年起成為費森尤斯醫療保健集團的一部分,新的設計也使公司在視覺上與其母公司的品牌形象保持一致。www.xenios-ag.com有了這個新結構的網站,…閱讀更多
沒有肺的六天——未來醫療技術展望
2017年4月12日
加拿大人梅利莎•伯努瓦(Melissa Benoit)在沒有肺的情況下活了6天的戲劇性故事,展現了醫療技術史上的新篇章。替代肺功能的新生iLA膜肺是XENIOS AG公司的產品。作為多倫多XOR實驗室的戰略合作夥伴,兩家公司都希望合作來徹底改變……閱讀更多
白皮書-案例研究
視頻
文章
博客
此選項卡的內容基於公司簡介。
如果您以公司用戶身份登錄,或者您是公司編輯,那麼您可以在這裏編輯公司簡介。